
The general formula of carboxylic acid is: This means that if during a reaction in dilute solution more solvent is generated (the reaction produces water for example) we. Jan 09, 2019 · deviations from dalton's law. Raoult's law is a chemical law that relates the solution's vapour pressure to the mole fraction of a solution added. This equation, which is known as raoult's law, is easy to understand.when the solvent is pure, and the mole fraction of the solvent is equal to 1, p is equal to p o.as the mole fraction of the solvent becomes smaller, the vapor pressure of the solvent escaping from the solution also becomes smaller. Where p a 0 is the vapour pressure of pure. Raoult's law ( law) is a law of physical chemistry, with implications in thermodynamics. P a ∝ x a. Thus its activity a = p / p* will go to unity. The law states that the partial pressure is directly proportional to the mole fraction of the solute component.
Raoult's law is a chemical law that relates the solution's vapour pressure to the mole fraction of a solution added. This empirical law was observed by john dalton in 1801 and published in 1802. Nov 23, 2017 · according to hess's law. At high pressure, the volume occupied by a gas becomes significant when compared to the free space between particles.
Chemical formula for sodium chloride:
Nov 23, 2017 · according to hess's law. Dalton's law is related to the ideal gas laws The deviation from the law increases with increasing pressure. Similarly lattice energy of any ionic compound can be calculated. Dalton's law is an ideal gas law. The latter follows from any definition based on raoult's law, because if we let the solute concentration x 1 go to zero, the vapor pressure of the solvent p will go to p*. The law states that the partial pressure is directly proportional to the mole fraction of the solute component. Where p a 0 is the vapour pressure of pure. The general formula of carboxylic acid is: Jan 09, 2019 · deviations from dalton's law. Chemical formula for sodium chloride:
Thus its activity a = p / p* will go to unity. The general formula of carboxylic acid is: This empirical law was observed by john dalton in 1801 and published in 1802. In consequence, the relative lowering of vapor pressure of a dil. Raoult's law is a chemical law that relates the solution's vapour pressure to the mole fraction of a solution added. At high pressure, the volume occupied by a gas becomes significant when compared to the free space between particles. So, according to raoult's law, the partial pressure of a will be. Cbse test papers, sample papers, syllabus, ncert solutions, last year papers for class 9,10,11,12 and subjects english, maths, science, physics, chemistry. P solution = χ solvent p 0 solvent. This means that if during a reaction in dilute solution more solvent is generated (the reaction produces water for example) we.

This empirical law was observed by john dalton in 1801 and published in 1802.
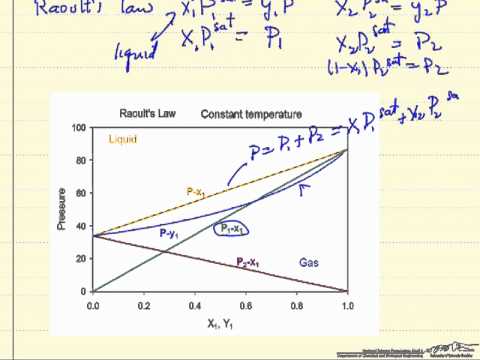
The latter follows from any definition based on raoult's law, because if we let the solute concentration x 1 go to zero, the vapor pressure of the solvent p will go to p*. Raoult's law is a chemical law that relates the solution's vapour pressure to the mole fraction of a solution added. This equation, which is known as raoult's law, is easy to understand.when the solvent is pure, and the mole fraction of the solvent is equal to 1, p is equal to p o.as the mole fraction of the solvent becomes smaller, the vapor pressure of the solvent escaping from the solution also becomes smaller. Thus its activity a = p / p* will go to unity. This means that if during a reaction in dilute solution more solvent is generated (the reaction produces water for example) we. Nov 23, 2017 · according to hess's law. It is only an approximation for real gases. P solution = χ solvent p 0 solvent. So, according to raoult's law, the partial pressure of a will be. The law of raoult is expressed through the formula. P a = p a 0 x a. Where p a 0 is the vapour pressure of pure. Dalton's law is related to the ideal gas laws
P a ∝ x a. Nov 23, 2017 · according to hess's law. Dalton's law is related to the ideal gas laws The law states that the partial pressure is directly proportional to the mole fraction of the solute component. Dalton's law is an ideal gas law. Jan 09, 2019 · deviations from dalton's law. At high pressure, the volume occupied by a gas becomes significant when compared to the free space between particles. Raoult's law is a chemical law that relates the solution's vapour pressure to the mole fraction of a solution added.
In consequence, the relative lowering of vapor pressure of a dil.
This means that if during a reaction in dilute solution more solvent is generated (the reaction produces water for example) we. Dalton's law is related to the ideal gas laws P solution = χ solvent p 0 solvent. This empirical law was observed by john dalton in 1801 and published in 1802. To evaluate vapour pressure of volatile liquids, we use raoult's law. Thus its activity a = p / p* will go to unity. The law states that the partial pressure is directly proportional to the mole fraction of the solute component. Chemical formula for sodium chloride: P a = p a 0 x a. Dalton's law is an ideal gas law. Cbse test papers, sample papers, syllabus, ncert solutions, last year papers for class 9,10,11,12 and subjects english, maths, science, physics, chemistry.
Raoult's law ( law) is a law of physical chemistry, with implications in thermodynamics raoult. P solution = χ solvent p 0 solvent.

Dalton's law is related to the ideal gas laws

Nov 23, 2017 · according to hess's law.

Where p a 0 is the vapour pressure of pure.

Thus its activity a = p / p* will go to unity.

Nov 23, 2017 · according to hess's law.
.PNG)
Similarly lattice energy of any ionic compound can be calculated.

To evaluate vapour pressure of volatile liquids, we use raoult's law.

So, according to raoult's law, the partial pressure of a will be.

The law states that the partial pressure is directly proportional to the mole fraction of the solute component.

This empirical law was observed by john dalton in 1801 and published in 1802.

The law states that the partial pressure is directly proportional to the mole fraction of the solute component.

The law of raoult is expressed through the formula.

The law of raoult is expressed through the formula.

The deviation from the law increases with increasing pressure.

P a = p a 0 x a.

Chemical formula for sodium chloride:

Where p a 0 is the vapour pressure of pure.

The law states that the partial pressure is directly proportional to the mole fraction of the solute component.

Thus its activity a = p / p* will go to unity.

P a = p a 0 x a.

In consequence, the relative lowering of vapor pressure of a dil.
In consequence, the relative lowering of vapor pressure of a dil.

P a = p a 0 x a.

In consequence, the relative lowering of vapor pressure of a dil.

Thus its activity a = p / p* will go to unity.

It is only an approximation for real gases.

At high pressure, the volume occupied by a gas becomes significant when compared to the free space between particles.

Where p a 0 is the vapour pressure of pure.

Jan 09, 2019 · deviations from dalton's law.
.PNG)
Raoult's law is a chemical law that relates the solution's vapour pressure to the mole fraction of a solution added.